EUROPE
EU-IVDR Compliance and Services
The IVDR is very much in its infancy and all stakeholders need to be prepared for it to evolve over the coming years.
There is an enormous amount of work required to transition from the IVDD to the IVDR, manufacturers need to recognize that the IVDR is more complex and detailed and will, therefore, require more resources to maintain as well as implement. In addition, whilst the requirements have been defined in the text the expectations are still evolving, experience from the medical device sector where there were five evolutions suggests expectations will continue to increase and files will need to be remediated.
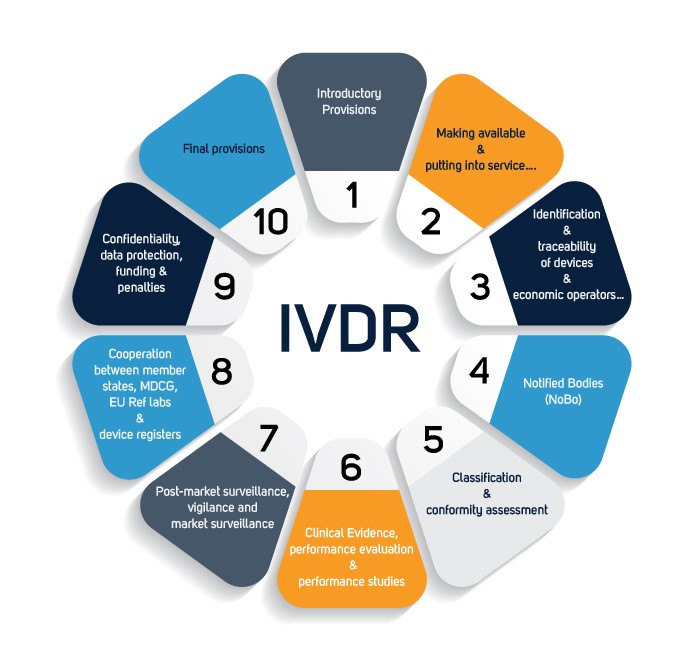
EU-IVDR
Did you check your EU-MDR compliance?
Download the Whitepaper '10 Things You Need to Know About the New IVDR'
EU-MDR Compliance Check
EU-IVDR
Services and training
Qserve has developed services and training to not only teach the requirements but educate our clients through hands-on workshops where they gain a greater understanding about what the requirements are, industry best practice and how to apply these to their own organization. We know getting a budget to travel is difficult so some of these courses can be web-based and can be followed up with on-site workshops specific to your company.
EU-IVDR
IVDR Experts
Qserve is here to support you on this journey, using our inside track to understand the requirements and keep you ahead of the curve. Our staff have been intimately involved with the development of the new IVD Regulations and can share this inside knowledge with our clients to help create submissions in a Notified Body friendly format and can guide you through the implementation process from concept to market access.