Qserve is the largest consultant company in the EU that is 100% focused on medical devices and in-vitro diagnostics
Successfull MDR Submissions
510(K) Approvals
PMCF Surveys Performed
Trusted by the industry













Featured content
Stay ahead with Qserve InSight
We understand that staying up-to-date with ever-changing regulations can be daunting and time-consuming. That's why we've developed Qserve InSight, our innovative tool designed to streamline your compliance processes and provide you with the information you need to stay ahead.
Introducing Qserve Learn!
Qserve Learn is a flexible and comprehensive online training platform designed to equip clinical, regulatory, and quality professionals in the Medical Device and IVD sector with expert-led courses, virtual training, and on-demand resources to support their professional growth.
6th Qserve MedTech Conference
We’re pleased to announce the return of the Qserve MedTech Conference, a must-attend event for MedTech professionals navigating an evolving global regulatory landscape. We'll be hosting the conference on 11-12 November, 2026 in Amsterdam.
We believe every patient deserves access to safe and innovative medical technologies. Qserve’s mission is to support MedTech companies worldwide in achieving regulatory compliance and clinical success—so that life-changing devices reach those who need them most.
Let's talk!
Contact us with your request or question
Contact us with your request or question
Learn more about Qserve
Your partner with a practical approach balancing business needs and regulatory compliance.
Be part of Qserve
Do you want to contribute to improving patient safety? Check our career page and join the Qserve team
Ready to secure compliance and market access?
Whether you’re introducing a breakthrough innovation or managing the compliance of legacy devices, Qserve is here to guide you throughout the entire life cycle of your device.
Contact us today to discuss how we can support your needs.
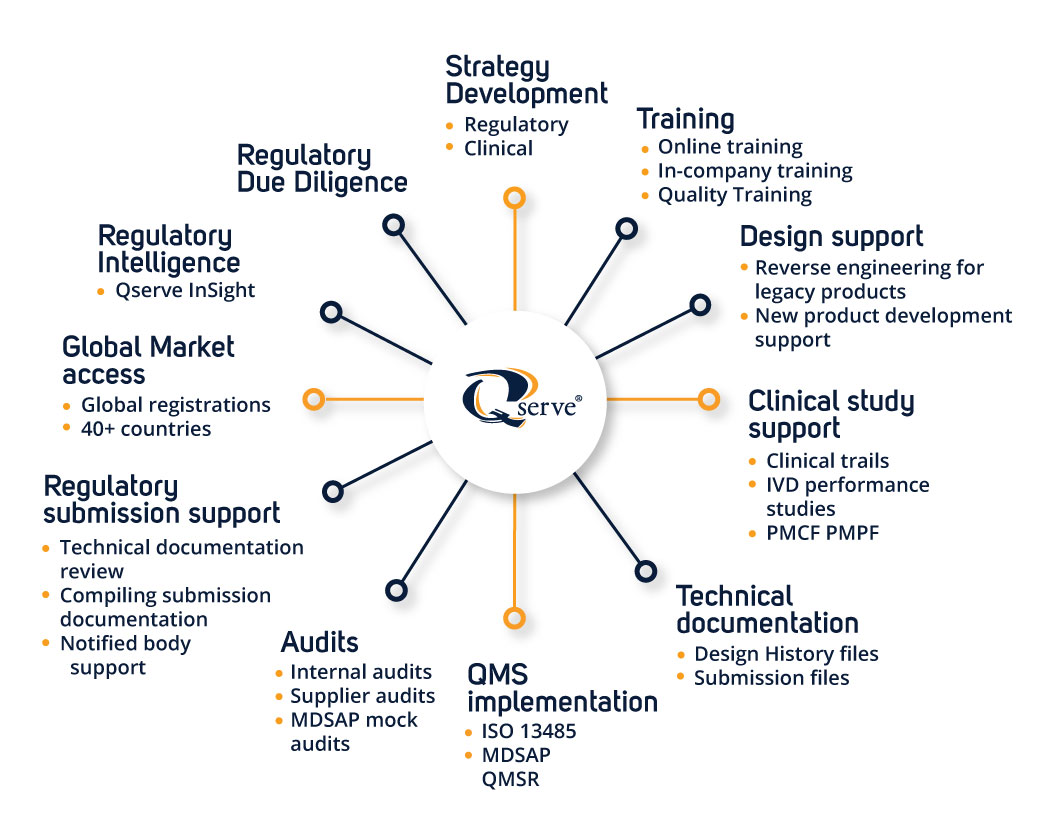
Global Regulatory Expertise with Local Presence
Qserve operates internationally with offices in the US, Europe, and China. This enables us to provide deep local regulatory knowledge while maintaining a global compliance strategy—crucial for companies targeting multiple markets (e.g., FDA, EU MDR/IVDR, NMPA).
Specialized in MedTech Only
Qserve focuses exclusively on medical devices and IVDs. Our consultants are engineers, scientists, and clinicians with deep industry-specific knowledge, offering tailored solutions rather than generic regulatory advice.
Hands-On Industry Experience
Many Qserve consultants come directly from industry roles (RA/QA, Research and Development, Clinical), bringing real-world understanding of what it takes to get products to market efficiently and maintain compliance throughout the lifecycle.
Integrated Services Across the Product Lifecycle
From strategic regulatory planning, clinical evaluations, and quality system implementation to post-market surveillance and due diligence, Qserve offers end-to-end support, reducing the need for multiple consultants.
Strong Track Record with Notified Bodies and the FDA
Qserve maintains trusted relationships with notified bodies and has significant experience with FDA submissions (510(k), PMA, De Novo), increasing the probability of successful and timely approvals.
Efficient Clinical Research
Qserve inhouse CRO is particularly skilled in supporting manufacturers with a practical approach. Qserve combines regulatory strategy, determining the need and depth of the data, in combination with an experienced CRO team. Covering all phases of clinical development, including PMCF surveys.
Data-Driven Clinical & Performance Evaluation
Qserve has robust in-house clinical and biostatistics expertise to design and execute clinical studies evaluation reports (CERs) and performance evaluation reports (PERs) that meet the rigorous demands of MDR and IVDR.
Rapid Support for M&A and Regulatory Due Diligence
Qserve frequently supports investors and MedTech firms in mergers, acquisitions, and licensing deals by assessing technical documentation, quality systems, and regulatory risks—vital for deal valuation and timelines.
Training and Knowledge Transfer
Beyond consulting, Qserve emphasizes client empowerment. We offer tailored training programs and actively transfer knowledge to internal teams, enabling clients to build stronger in-house regulatory capabilities.
"
"We had our MDR audit completed today with zero nonconformances. Thank you for helping us become better prepared for the audit than we were before you completed the internal audit."
- SME, United States
"
"Thank you very much for organizing all the necessary files for us. You helped a lot. I am impressed by how fast and efficient everything works with you."
- Multinational, Switserland
"
"I Wanted to say a huge thank you on behalf of our team for the training. It was super helpful, thanks for pivoting around our questions and the interaction!"
- Multinational, Ireland
"
"I would like to inquire about contracting with Qserve again for our Detector-Point product family Clinical Evaluation Plan and Report. We have worked with Qserve on 3 previous projects and have been greatly impressed with the results."
- SME, India
Latest blogs
-1.png)
Learn where Medical Device Start-Ups go wrong and how to avoid these pitfalls.
.png)
Learn about the UKRP appointment and registration process, the importance of proper documentation, and manufacturers' obligations from a UKRP perspective.

Clinical data quality drives every study decision. Learn how Qserve handles structured data management and thorough UAT to prevent delays and surprises.
