Regulatory IVD services
IVD Regulatory consulting
Are you developing or selling in vitro medical devices or companion diagnostics? Qserve helps IVD manufacturers with IVD regulatory consulting services in all product life cycle phases to achieve IVD compliance and facilitate market access in multiple regions globally. Qserve's IVD experts all have industry experience and can support every step in the design and development process, such as regulatory submissions, US FDA submissions, pre-submissions, the EU-IVDR, including the transition from IVDD to IVDR, and on-market support.
Many manufacturers are changing their market entry strategy and will enter the US market first but want to repurpose data for Europe. Whilst the regulatory systems are very different, there is commonality, particularly if guidance such as Clinical Laboratory Standard Institute (CLSI) guidance is applied in design and development. Our team can help write submissions using this data to support global submissions.

Regulatory, Quality, and Clinical Services
How can we help?
Qserve is an all-service IVD consultancy that can provide IVD regulatory consulting services throughout the product life cycle. Whether you are a start-up company, a small-medium enterprise (SME), or an established or multinational company, Qserve has services that can support your needs, whether you need expert knowledge or resources to augment your own quality and regulatory groups.
New device
- Discuss strategy, first market entry, regulatory challenges and opportunities.
- Focus on the intended purpose as this drives the design & development plan.
- Explain the requirements, evaluate existing data, and how to fill the gaps.
- Explain how to implement the QMS using a phased approach so essential procedures are in place at the right time while also spreading your budget.
New markets and Commercialization
- Strategy support for new market entry via design changes, changes in intended purpose, market repositioning, or geographical expansion.
- Global presence with offices in the US, Europe, UK, and China, facilitating regulatory submissions for the FDA, EU-IVDR, China, and worldwide.
- Market access is aided by EU Authorized Representation, US Agent, UK Responsible Person, China Agent services, and global access through partnerships.
- Comprehensive training suite covering IVD regulations and QMS implementation.
Design and development process
Product Life Cycle Support
- Qserve has real-world experience and provides a practical approach to all steps of the design and development process for IVDs.
- Our IVD team can help start-up projects navigate all elements of the process.
- We can also help existing companies navigate the impact of design changes on regulatory submissions.
- We can discuss science with your IVD teams and converse with your quality and regulatory professionals to find the most effective and compliant solutions.
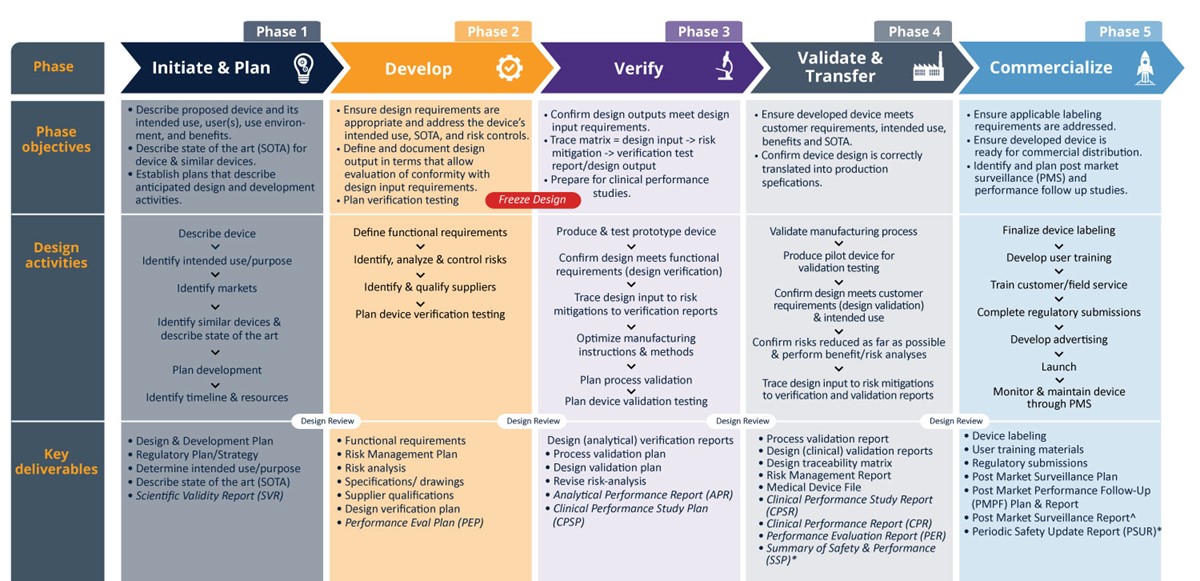
Download the IVD design and development process to learn more about the steps in the product life cycle.
Download IVD leaflet