
About Qserve Group
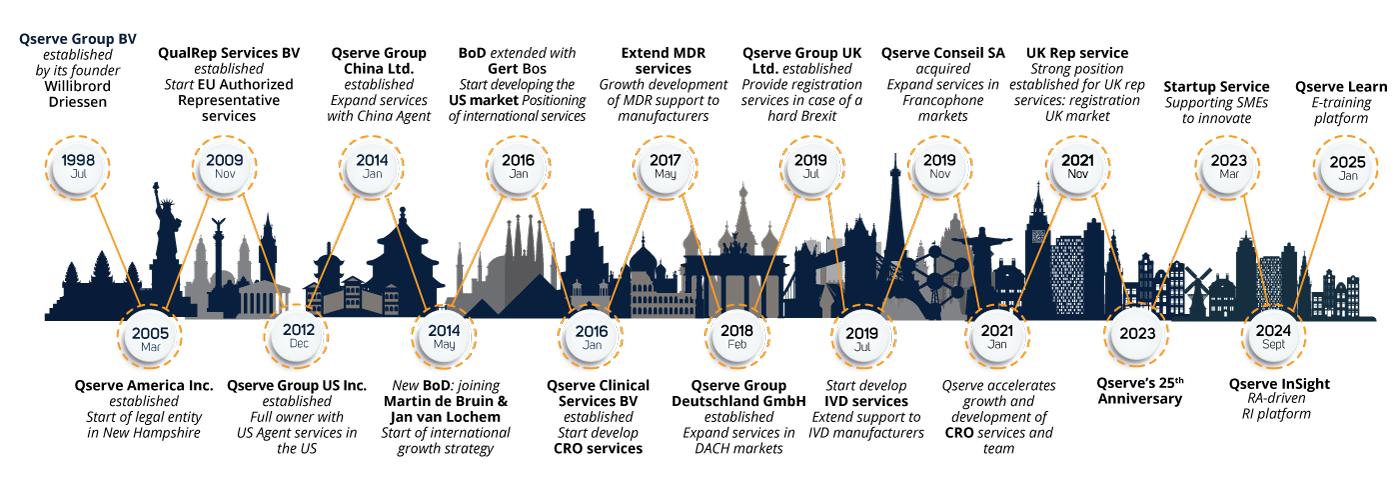
Qserve Group was founded in 1998 and started with a small group of regulatory experts. Since 2014, three new shareholders joined the Board of Directors with the strong aim to continue in line with the global vision.
Our mission is to help improve patient safety and health by supporting the advancement of biomedical technology. We assist medical device and in-vitro diagnostics manufacturers worldwide with market access, medical device approvals, clinical trials and achieving and maintaining regulatory compliance.
We strive to do this as your partner with a practical approach balancing business needs and regulatory compliance.
We are passionately working for our customers on regulatory, quality, and clinical challenges and see it as our responsibility to create a diverse global team of professionals who share the same passion for medical device technology, a high standard of quality, competence, and customer service, and enjoy the fun of working on this together every day. Our people make our firm.

Our Therapeutic Specialties
We are widely recognized for our "practical" mindset—many of our consultants come from the MedTech industry, often having worked in regulatory, quality, clinical, or R&D roles or having been employed by notified bodies. This makes their advice practical, implementation-ready, and grounded in operational reality, not just theoretical knowledge.




The board of Qserve Group
Qserve Group was founded in 1998 by Willibrord Driessen with a small team of regulatory experts. Since 2014, three new shareholders joined the Board of Directors, sharing Willibrord's global vision. Today, Qserve is a well-known global player in the medical device industry, with offices in Europe, the United States, and China, serving manufacturers worldwide.
The Board of Qserve Group includes Jan van Lochem, Martin de Bruin, and Gert Bos. They are committed to expanding Qserve Group and Qserve CRO into a top global medical device consulting group. We specialize in regulatory and clinical affairs, Regulatory Intelligence, Quality Assurance, Clinical Research and Training to help medical devices and diagnostics gain registration, approval, and market access worldwide.

Over the past 27 years, Qserve has developed into a global company where the team combines their regulatory knowledge and experience in the medical device and in vitro diagnostic industry, sharing more than 500 years' worth of combined expertise in the medical field.
The historical development shows Qserve makes you your global partner for Regulatory Compliance all over the world.
We have 7 offices worldwide
Get in touch to learn more





















%20Huang.png)







.png)










%2c%20CLS(NCA).png)

.png)










