
European Authorized Representative
Qserve acts as your trusted EU legal representative for medical devices and IVDs, ensuring MDR/IVDR compliance and smooth access to the European market.
EU Authorized Representative (EC REP)
The European Union requires any non-eu manufacturer who intends to sell their devices in the EU to designate a sole authorized representative (EC REP). The European Authorized Representative will represent your company to the national authorities and shall register your devices in the electronic system before commercialization. It is the liaison between a manufacturer and a national competent authority. The use of name and registered address is on all product related labelling.
With Qserve as your EU Authorized Representative, you secure a strategic partner committed to protecting your interests in the European marketplace while you focus on innovation and growth.
Responsibilities of the EU Rep
-
Access and availability of your most up to date technical file
-
Act within 24 hours in case of incidents and Field Safety Corrective Action (FSCA) reporting.
-
First point of contact for the competent authority
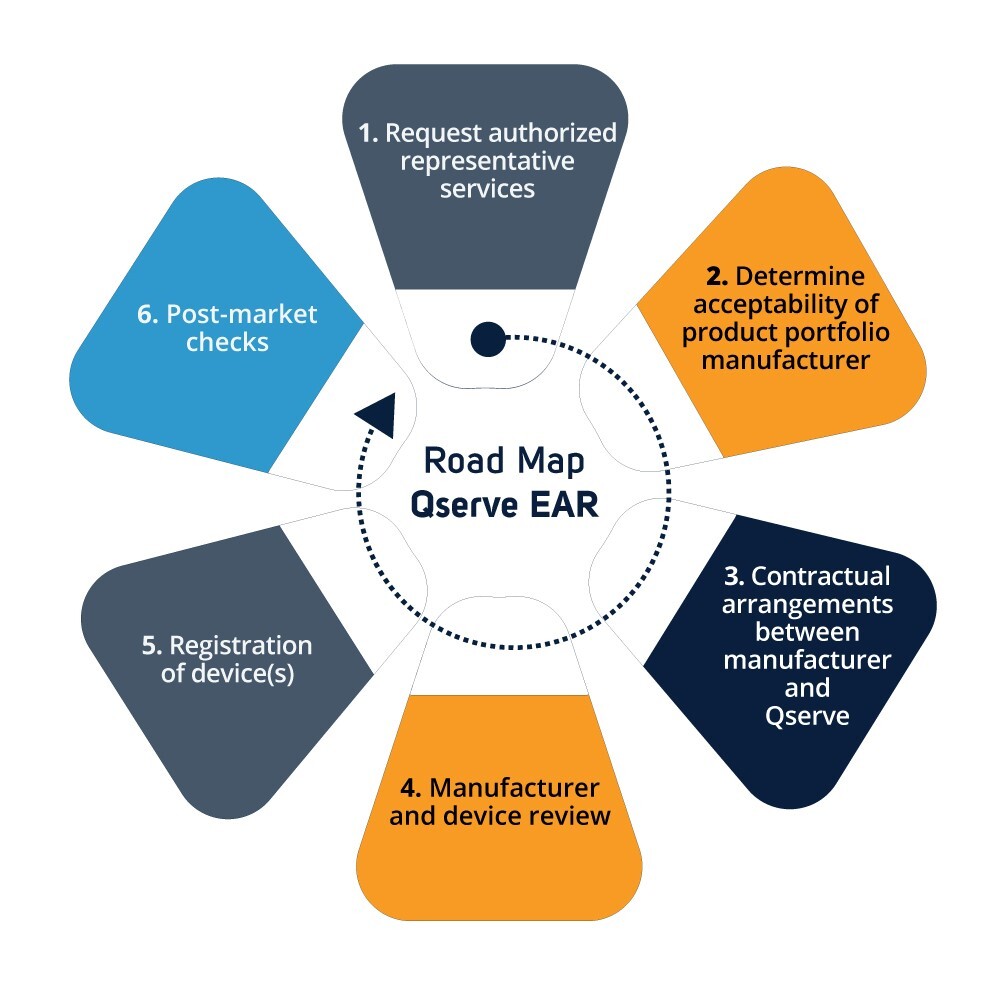
Due to the increased liability under the MDR/IVDR, Qserve has updated her processes and systems to meet the requirements underlying article 11. Qserve will review your technical documentation and provide support where necessary. Together we ensure that your documentation complies with the regulations. Once completed, your devices will be registered.
Depending on the classification of the device the type of review may vary. For Class I devices the manufacturer has to self-declare the conformity to the MDR/IVDR. For Class II and III, there is Notified Body involved, and only after approval (CE Certificate) the EAR can register the devices.
Legally Established in the EU
Qserve is a fully registered and compliant legal entity in the EU, meeting all MDR and IVDR requirements for Authorized Representatives.
Official Point of Contact for Regulatory Authorities
We act as your formally appointed EU representative, serving as the direct link between your company and the European Competent Authorities.
Independent and Trusted Partner
As an independent organization, Qserve provides reliable and unbiased support, always prioritizing your compliance and market success.
Advice on registration strategies
We guide you through the most efficient regulatory pathways for CE marking and EU market access.
Ensuring compliance with all EU regulatory requirements
We help you meet the obligations set by EU legislation, including vigilance, documentation, and registration.
Experienced partner for global manufacturers
From startups to global enterprises, Qserve has supported hundreds of manufacturers in navigating the complex EU regulatory landscape.

Download the EU Representative brochure to learn how Qserve can act as your EU Rep.
The EU Rep (EC Rep | EAR) will represent your company to the national authorities and shall register your devices in the electronic system before commercialization.