
Label here
Regulatory compliance for Taiwan
Expert support for Taiwan medical device compliance, guiding you through registration requirements.
Regulatory compliance for Canada
Taiwan agent representation
Taiwan agent representation
Taiwan’s Ministry of Health and Welfare (MOHW) is the government ministry responsible for the country’s healthcare regulations. The MOHW has a broad mandate to improve healthcare quality, prevent and control infectious diseases, ensure food and drug safety, and facilitate technological development.
Within the MOHW, the Taiwan Food and Drug Administration (TFDA) manages the regulatory system for the safety and quality of food, drugs, medical devices, and cosmetics. The TFDA drafts and implements regulations, grants product registration and clinical trial approvals, monitor manufacturing and importation and conducts safety surveillance activities on health products.
In May 2021, Taiwan announced a new Medical Devices Act, which changes how medical device products are classified, groups products by risk and category, and adjusts registration fees. It also reinforces post-market surveillance of device safety and quality by mandating regulations for product recalls.
Get Support Now

Registration routes in Taiwan
Medical Device and IVD registration
Medical Device
Pre-market approval is necessary for all classes of medical devices before Taiwanese market entry. The TFDA issues a "medical device permit license upon product registration approval."
If no similar devices have previously been approved by the TFDA, your device will be considered a PMA device in Taiwan. This is the case even if your product has a Substantially Equivalent (SE) device approved by the U.S. FDA.
Class II and III devices must have approval from their home country before Taiwanese registration.
Class I sterile, Class I measuring, Class II, and Class III medical devices must comply with GMP requirements before the TFDA grants product licenses.
TFDA-authorized auditing organizations perform on-site inspections for local manufacturers by GMP and review quality system documentation (QSD) provided by foreign manufacturers.
Before a medical device can be sold in Taiwan, Quality System Documentation (QSD) registration for the manufacturing facility is required in addition to medical device registration. QSD registration is only waived for Class I (non-sterile) medical devices. A QSD license (received upon QSD registration approval) in Taiwan is similar to a Good Manufacturing Practice (GMP) for medical devices.
IVD
The registration process for IVDs in Taiwan, overseen by the TFDA, varies according to the device's risk class, similar to that for medical devices.
Class I and II IVDs: These devices do not require registration and are subject to less stringent regulatory requirements than higher-risk devices.
Class III and New IVDs: These devices must undergo a two-stage review process, including Administrative and Technical Reviews.
The Role of a Taiwan Agent in Medical Device Registration
Regulatory timelines and fees for Taiwan
Class I (non-sterile)
- Class I medical devices without brand names (e.g., surgical instruments or power accessories) usually follow a simple self-declaration process in Taiwan.
- Timeline: The Class I registration approval process takes about 3 months.
- A list of Class I devices may be registered through the listing process outlined in Article 25 of the Medical Devices Act, effective October 1, 2021. The list contains 68 device codes.
Important links and guidance documents
Label here
Local representative
To sell medical devices or in vitro diagnostics (IVDs) in Taiwan, manufacturers without a local office must appoint an in-country regulatory representative, a Taiwan Agent. The Taiwan Agent is your liaison with the Taiwan Food and Drug Administration (TFDA) and is critical in ensuring compliance with Taiwanese regulations.
Your Taiwan Agent must:
- Be a legally established entity in Taiwan.
- Hold a Pharmaceutical Sales License to perform regulatory activities.
The Taiwan Agent provides comprehensive regulatory support, including:
- Registration Submission: Preparing and submitting all necessary documentation to the TFDA on your behalf.
- QSD Approval: Assisting with obtaining the Quality System Documentation (QSD) Approval Letter, which is mandatory for Class II, Class III, and specific Class I devices.
- Regulatory Liaison: Acting as the primary regulatory connection between your company and the TFDA, facilitating communication and compliance.
- SAE Reporting: Managing Serious Adverse Event (SAE) reporting should issues arise with your device.
- License Maintenance: Ensuring the QSD License and device registration remain current.
- Medical Device Product License Management: Holding your TFDA-approved Medical Device Product License, a requirement for all registered devices.
Your Taiwan Agent’s details must appear on the Chinese label of your device, along with the registration number. Additionally, distributors typically add their information using a sticker to comply with local practices.
The Taiwan Agent’s role is vital for navigating the complex regulatory landscape in Taiwan. They ensure your device meets all TFDA requirements, maintain your product’s compliance, and act as your local point of contact for regulatory issues.
Manufacturers can confidently expand their business into the Taiwanese market by appointing a reliable Taiwan agent, ensuring compliance and a smooth approval process.
Contact us to learn how Qserve can assist you in appointing a Taiwan agent and managing your regulatory needs in Taiwan.
Contact Us
Registration routes in Canada
Regulatory Process
Should the manufacturer of Class I medical devices or in vitro diagnostic devices aim to market their products directly in Canada without utilizing a distributor, they are obligated to obtain a Medical Device Establishment License (MDEL). If the manufacturer opts for distribution through one or more distributors within Canada, they must also undergo the application process for an MDEL. Put differently, distributors and importers based in Canada must apply for an MDEL, irrespective of the risk classification of the medical device or in vitro diagnostic device.
For manufacturers dealing with Class II-IV devices intending to sell their products, applying for an MDL is the prerequisite. The key distinction between an MDEL and an MDL lies in the fact that the MDL constitutes product approval, while the MDEL serves as a permit for the company, distributor, or importer itself.
In obtaining an MDL, manufacturers are required to demonstrate certification under the ISO 13485 quality management system through the Medical Device Single Audit Program (MDSAP). This certification must align with the specifications outlined in the Health Canada Medical Regulations.
Both license application procedures require submitting documents in electronic format to Health Canada, following the submission requirements set by Health Canada.
Guidence documents
Market Access
More information
Get in touch for more information about Taiwan Market Access
Get in touch for more information about Taiwan Market Access
medical_services
Medical device classification in Taiwan
The Taiwanese regulatory system classifies devices as Class I, II, and III, based on the US FDA medical device classification scheme.
- Class I�� Device: Low Risk
- Class II Device: Moderate Risk
- Class III Device: High Risk
Learn how we can simplify the process of entering the Taiwanese medical device market.
timeline
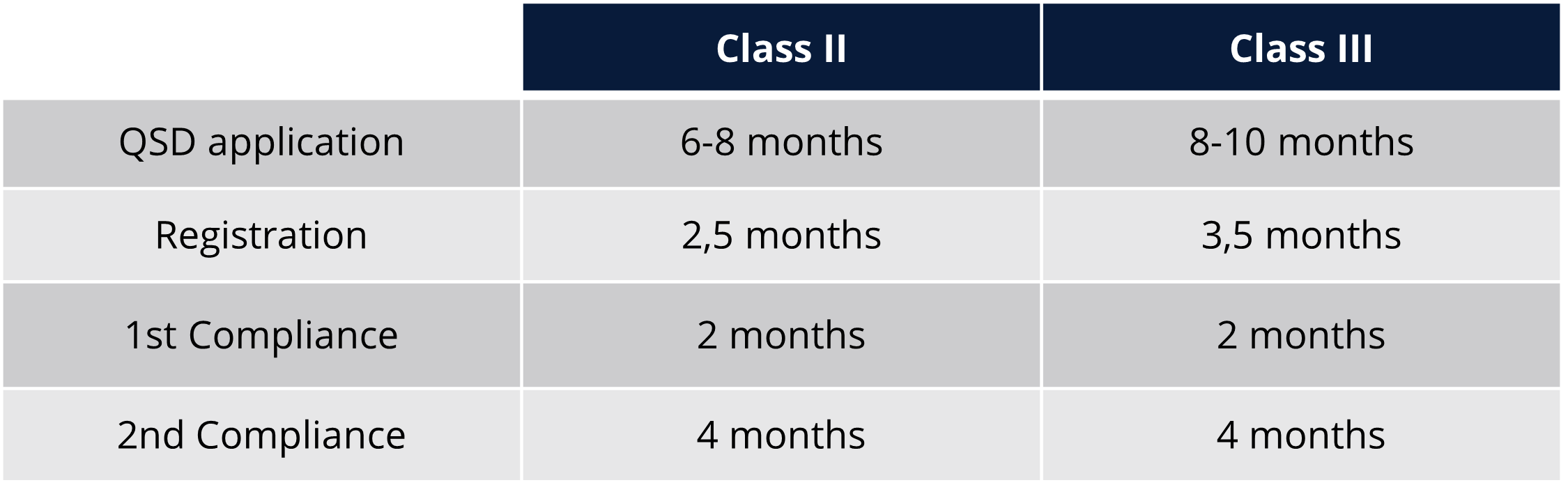
Regulatory timelines and fees
Navigating Taiwan’s medical device regulations can be challenging, but with Qserve, you gain a trusted partner to simplify the process. Whether you need help developing a strategy or managing regulatory requirements, we provide tailored support to ensure smooth market access in Taiwan.
Contact us today to learn how we can simplify the process of entering the Taiwanese medical device market.
Label here
Quality and Clinical Compliance Requirements
Quality and Clinical Compliance Requirements
Labeling
The label and IFU must be written in Chinese.
Technical Personnel requirements
Article 15 of the Medical Devices Act stipulates that manufacturers and importers of medical devices must appoint designated Technical Personnel, effective May 1, 2021. The Administrative Measures for Medical Device Technicians outlines the requirements and qualifications for medical device technical personnel required by all manufacturers and dealers. The new requirement will be subject to a three-year transition period.
UDI requirements
Taiwan has begun a phased implementation of UDI, including labeling and database reporting requirements. The “Requirements for Indicating the Unique Device Identifier on Medical Device Labels" came into force on 1 May 2021. Issuing agencies include GS1, HIBCC, and ICCBBA.
Get Support Now
server render fail/waiting for island-7f870ei1R0 (separate island render, inside)