Qserve ist das größte Beratungsunternehmen in der EU, das sich zu 100 % auf Medizinprodukte und In-vitro-Diagnostika konzentriert.
Erfolgreiche MDR-Einreichungen
510(K)-Zulassungen
Durchgeführte PMCF-Erhebungen
Vertrauen in der Industrie












Neueste Nachrichten
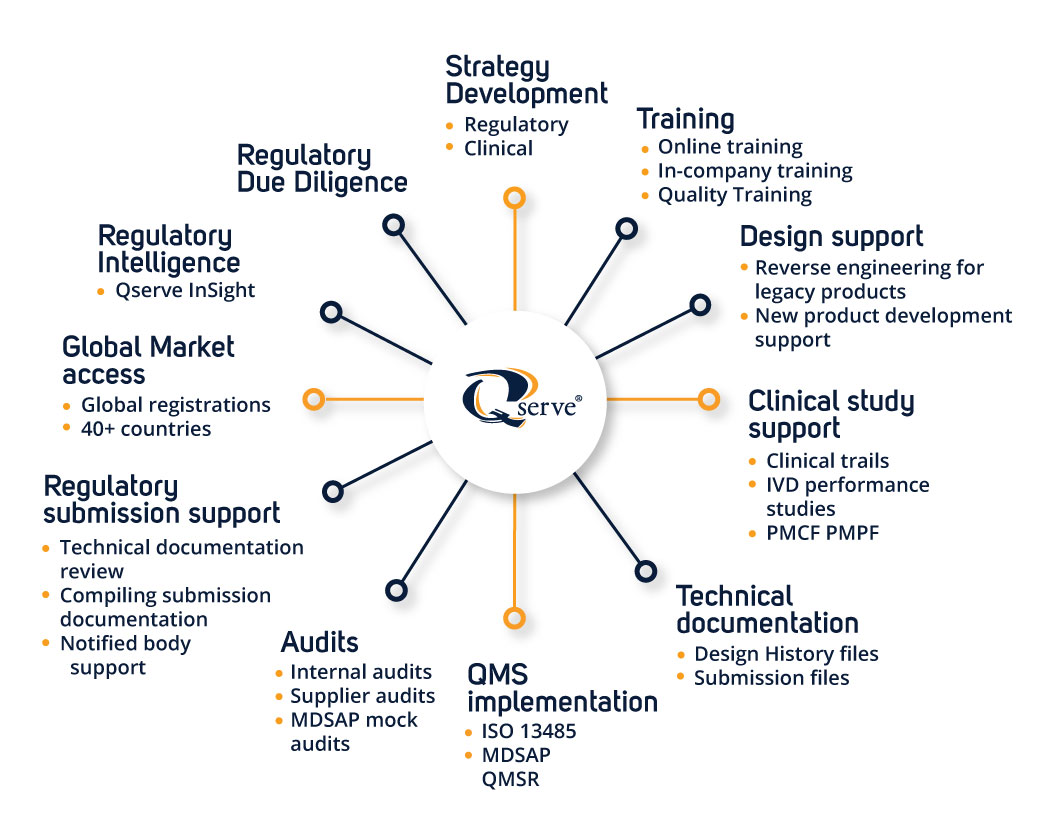
Mit Qserve InSight immer einen Schritt voraus
Wir wissen, dass es entmutigend und zeitaufwändig sein kann, mit den sich ständig ändernden Vorschriften Schritt zu halten. Deshalb haben wir Qserve InSight entwickelt, unser innovatives Tool, das Ihre Compliance-Prozesse rationalisiert und Ihnen die Informationen liefert, die Sie brauchen, um immer einen Schritt voraus zu sein.
Wir stellen vor: Qserve Learn!
Qserve Learn ist eine flexible und umfassende Online-Schulungsplattform, die Fachleuten aus den Bereichen Klinik, Regulierung und Qualität in der Medizinprodukte- und IVD-Branche von Experten geleitete Kurse, virtuelle Schulungen und On-Demand-Ressourcen bietet, um ihre berufliche Entwicklung zu unterstützen.
Fusionen und Übernahmen im Jahr 2025
Die regulatorische Due-Diligence-Prüfung ist bei Fusionen und Übernahmen im MedTech-Bereich unerlässlich. Erwerber müssen das regulatorische Risikoprofil des Zielunternehmens verstehen und die Risiken im Zusammenhang mit dem Geschäft bewerten.
Wir glauben, dass jeder Patient Zugang zu sicheren und innovativen medizinischen Technologien verdient. Qserve hat es sich zur Aufgabe gemacht, MedTech-Unternehmen weltweit bei der Einhaltung gesetzlicher Vorschriften und dem klinischen Erfolg zu unterstützen, damit lebensverändernde Medizinprodukten diejenigen erreichen, die sie am dringendsten benötigen.
Reden Sie mit uns!
Kontaktieren Sie uns mit Ihrer Anfrage oder Frage
Kontaktieren Sie uns mit Ihrer Anfrage oder Frage
Erfahren Sie mehr über Qserve
Ihr Partner mit einem praktischen Ansatz, der Geschäftsanforderungen und die Einhaltung von Vorschriften in Einklang bringt.
Werden Sie Teil von Qserve
Möchten Sie zur Verbesserung der Patientensicherheit beitragen? Besuchen Sie unsere Karriereseite und schließen Sie sich dem Qserve-Team an.
Sind Sie bereit, die Einhaltung von Vorschriften und den Marktzugang zu sichern ?
Ganz gleich, ob Sie eine bahnbrechende Innovation einführen oder die Konformität älterer Medizinprodukte verwalten wollen, Qserve begleitet Sie während des gesamten Lebenszyklus Ihres Geräts.
Kontaktieren Sie uns noch heute, um zu besprechen, wie wir Sie unterstützen können.
Globales regulatorisches Fachwissen mit lokaler Präsenz
Qserve ist international mit Niederlassungen in den USA, Europa und China tätig. Dadurch kann Qserve fundierte Kenntnisse der lokalen Vorschriften bieten und gleichzeitig eine globale Strategie zur Einhaltung der Vorschriften verfolgen - eine wichtige Voraussetzung für Unternehmen, die auf mehrere Märkte abzielen (z. B. FDA, EU MDR/IVDR, NMPA).
Nur auf Medizintechnik spezialisiert
Qserve konzentriert sich ausschließlich auf Medizinprodukte und IVDs. Die Berater sind Ingenieure, Wissenschaftler und Kliniker mit fundierten branchenspezifischen Kenntnissen und bieten maßgeschneiderte Lösungen statt allgemeiner regulatorischer Beratung.
Praktische Erfahrung in der Industrie
Viele Qserve-Berater kommen direkt aus der Industrie (RA/QA, Forschung und Entwicklung, klinische Forschung) und bringen ein praktisches Verständnis dafür mit, was nötig ist, um Produkte effizient auf den Markt zu bringen und die Konformität während des gesamten Lebenszyklus zu gewährleisten.
Integrierte Dienstleistungen über den gesamten Produktlebenszyklus
Von der strategischen Zulassungsplanung über klinische Bewertungen und die Implementierung von Qualitätssystemen bis hin zur Überwachung nach der Markteinführung und Due-Diligence-Prüfungen bietet Qserve eine durchgängige Unterstützung, die den Bedarf an mehreren Beratern reduziert.
Starke Erfolgsbilanz bei benannten Stellen und der FDA
Qserve unterhält vertrauensvolle Beziehungen zu den benannten Stellen und verfügt über umfangreiche Erfahrungen mit FDA-Anträgen (510(k), PMA, De Novo), was die Wahrscheinlichkeit einer erfolgreichen und rechtzeitigen Zulassung erhöht.
Effiziente klinische Forschung
Das hauseigene CRO von Qserve ist besonders gut darin, Hersteller mit einem praktischen Ansatz zu unterstützen. Qserve kombiniert eine regulatorische Strategie, die den Bedarf und die Tiefe der Daten bestimmt, mit einem erfahrenen CRO-Team. Wir decken alle Phasen der klinischen Entwicklung ab, einschließlich PMCF-Erhebungen.
Datengestützte klinische und Leistungsbewertung
Qserve verfügt über ein solides internes klinisches und biostatistisches Fachwissen, um Berichte zur Bewertung klinischer Studien (CERs) und Leistungsbewertungsberichte (PERs) zu entwerfen und durchzuführen, die den strengen Anforderungen von MDR und IVDR entsprechen.
Schnelle Unterstützung für M&A und regulatorische Due Diligence
Qserve unterstützt häufig Investoren und MedTech-Firmen bei Fusionen, Übernahmen und Lizenzgeschäften, indem es die technische Dokumentation, die Qualitätssysteme und die regulatorischen Risiken bewertet, die für die Bewertung des Geschäfts und den Zeitplan entscheidend sind.
Ausbildung und Wissenstransfer
Neben der Beratung legt Qserve großen Wert auf die Befähigung der Kunden. Qserve bietet maßgeschneiderte Schulungsprogramme an und gibt sein Wissen aktiv an interne Teams weiter, so dass die Kunden in der Lage sind, stärkere interne regulatorische Kapazitäten aufzubauen.
"
"We had our MDR audit completed today with zero nonconformances. Thank you for helping us become better prepared for the audit than we were before you completed the internal audit."
- SME, United States
"
"Thank you very much for organizing all the necessary files for us. You helped a lot. I am impressed by how fast and efficient everything works with you."
- Multinational, Switserland
"
"I Wanted to say a huge thank you on behalf of our team for the training. It was super helpful, thanks for pivoting around our questions and the interaction!"
- Multinational, Ireland
"
"I would like to inquire about contracting with Qserve again for our Detector-Point product family Clinical Evaluation Plan and Report. We have worked with Qserve on 3 previous projects and have been greatly impressed with the results."
- SME, India
